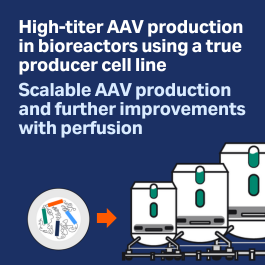
Robust and scalable AAV production
Read about the upstream process development and optimization of AAV production in batch and perfusion modes.
Adeno-associated virus (AAV) vectors have emerged as a promising tool for gene therapy, holding tremendous potential to treat a wide array of genetic disorders. However, the journey from lab to commercial production has not been without obstacles. In this video, we'll explore valuable insights into challenges and triumphs of AAV manufacturing.

Read about the upstream process development and optimization of AAV production in batch and perfusion modes.
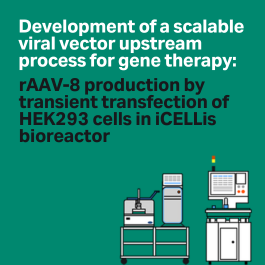
Read about the scalable upstream process we developed for rAAV8 in HEK293 cells.

Learn how we developed a scalable, cGMP compatible process in a stirred-tank, single-use bioreactor.

See how we achieved baseline separation of full and empty capsids with ~ 80% VG recovery and purity.
Adeno-associated viral (AAV) vectors are a common delivery system for gene therapies with advantages including enhanced safety compared to other vectors. A variety of AAV serotypes target different tissues to support a variety of therapeutic applications, but they all share common manufacturing challenges and solutions.
Why AAV?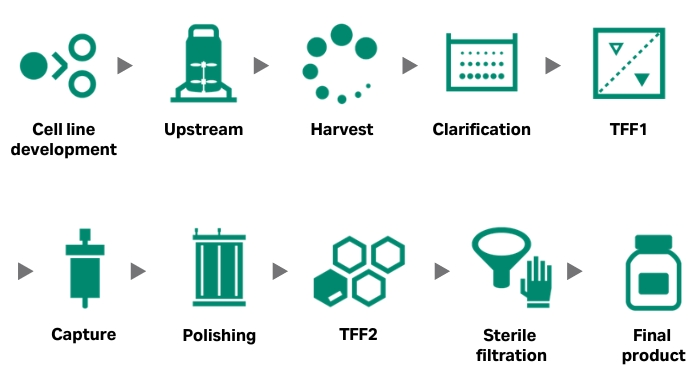
Use our know-how to simplify and accelerate your development and meet your process milestones.
Learn more
Prevalent indications are the next frontier for adeno-associated virus (AAV)-based gene therapy. Transient transfection is simple but not efficient or scalable enough. An ELEVECTA™ producer cell line contains all genes to produce rAAV; simply induce. The result is a simplified and robust upstream process that easily scales to treat hundreds of thousands.
Start now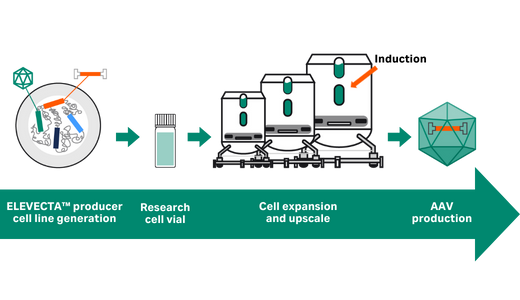

Technology for adherent cells used in several approved gene therapies.

Purify small quantities efficiently in a GMP environment.
Dextran surface extenders on this chromatography resin enhance separation of full and empty capsids.

Efficient, industrial-scale purification of AAV of several subclasses by affinity chromatography.